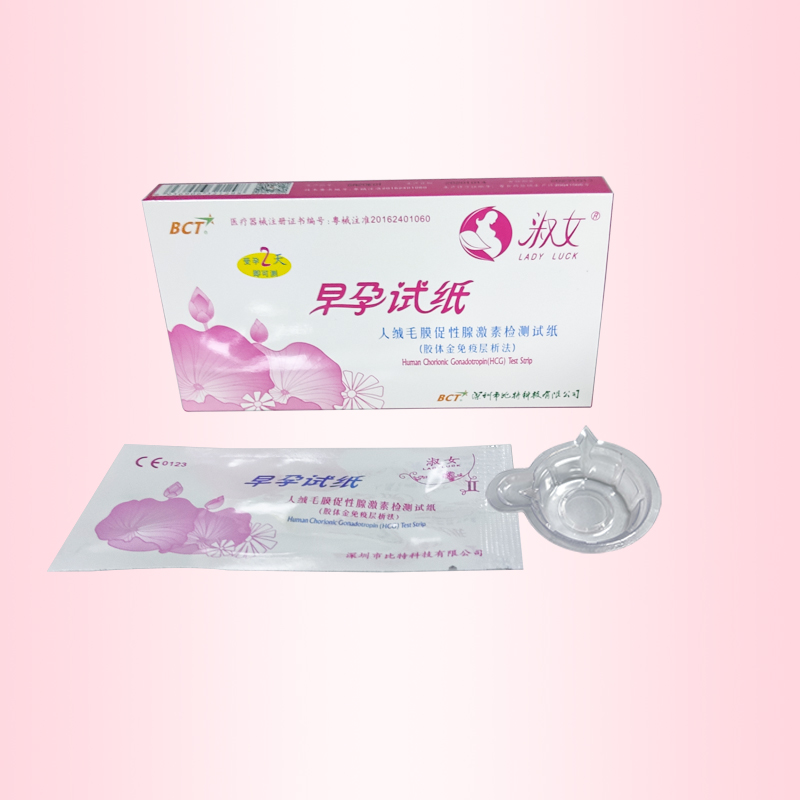
- Product Name: Pregnancy test strip
-
null(en)
型号规格 HCG-A02 1人份/盒
【Common name】 Human chorionic gonadotropin (HCG) test strip (colloidal
gold immunochromatography)
【English name】 Human Chorionic Gonadotropin Test Strip (colloidal gold immunochromatography)
【Product Name】 Pregnancy test cassette 1 test / box
【Main Composition】
From the test strip and its support composition, test strips on the main components are:
A) Human chorionic gonadotropin antibody (immobilized on a nitrocellulose membrane);
B) Anti-mouse IgG polyclonal antibody (immobilized on nitrocellulose membrane);
C) Colloidal gold-labeled anti-HCG monoclonal antibody (immobilized on glass fiber);
D) Other test strip supports.
Other test paper accessories, such as straws, desiccants and so on.
【Storage conditions and validity】
Stored at 2 to 30 degrees Celsius from dark, self-test qualified from the date of validity of 36 months, Kaifeng should be used immediately.
【Sample Requirement】
Any time the urine is suitable for this experiment, in the early stages of pregnancy, with the morning urine is the best.
【Testing method】
1. Collect urine from a clean, dry container (eg, such as an attached urine cup).
2. Use the aluminum foil bag to tear along the gap, remove the pregnancy test cassette, the pregnancy cassete flat; with a small straw suction urine sample 3 drops (about 0.2ml) added to the pregnancy test cassette sample hole .
3. Please 30 seconds to 5 minutes to observe the results in the observation window to 5 minutes to read the subject!
【Manufacturer】
BCT Systems Co., Ltd.
【English name】 Human Chorionic Gonadotropin Test Strip (colloidal gold immunochromatography)
【Product Name】 Pregnancy test cassette 1 test / box
【Main Composition】
From the test strip and its support composition, test strips on the main components are:
A) Human chorionic gonadotropin antibody (immobilized on a nitrocellulose membrane);
B) Anti-mouse IgG polyclonal antibody (immobilized on nitrocellulose membrane);
C) Colloidal gold-labeled anti-HCG monoclonal antibody (immobilized on glass fiber);
D) Other test strip supports.
Other test paper accessories, such as straws, desiccants and so on.
【Storage conditions and validity】
Stored at 2 to 30 degrees Celsius from dark, self-test qualified from the date of validity of 36 months, Kaifeng should be used immediately.
【Sample Requirement】
Any time the urine is suitable for this experiment, in the early stages of pregnancy, with the morning urine is the best.
【Testing method】
1. Collect urine from a clean, dry container (eg, such as an attached urine cup).
2. Use the aluminum foil bag to tear along the gap, remove the pregnancy test cassette, the pregnancy cassete flat; with a small straw suction urine sample 3 drops (about 0.2ml) added to the pregnancy test cassette sample hole .
3. Please 30 seconds to 5 minutes to observe the results in the observation window to 5 minutes to read the subject!
【Manufacturer】
BCT Systems Co., Ltd.
Registration, production Address: 4F&Right side of 2F, No.3
Lady Road,Fucheng, Longhua , Shenzhen.
Postal Code: 518110
【Medical Device Manufacturer License No.】
Guangdong Drug Administration Department Production Permit No.
20041005
【Medical device registration certificate No.】
Guangdong Device Registration No. 20162401060
【Technical requirements for medical instruments】
Guangdong Device Registration No. 20162401060